Covaxin Who Approval Date : Coronavirus India Approves Covid 19 Vaccines Covishield And Covaxin For Emergency Use The Hindu
Come 5 October Indians who took two shots of Bharat Biotechs Covaxin will know if they have to wait longer before they can travel abroad. All passengers from India who have received two doses of Covaxin at least 14 days before the estimated arrival date will now be able to travel to Oman without the.
The Strategic Advisory Group of Experts on Immunization SAGE will meet on October 5 to grant authorization to Covaxin.

Covaxin who approval date. The World Health Organisations approval for emergency use of Covaxin can come before the end of the month Dr VK Paul chairman of the National Expert Committee on. A WHO official has said the agencys assessment of made-in-India. October 20 2021 1058 AM IST.
Covaxin developed and made in India becomes the eighth Covid-19 vaccine approved by WHO Photo. WHOs emergency use approval to Covaxin delayed till October 5. Eager for WHO approval for Covaxin.
WHO an independent group of experts are scheduled to meet next week to carry out the risk. Though Covaxin was licenced by Indian regulators in January this year when the company began phase 2 studies WHO requires evidence from the phase 3 trial. By Anshul Dhamija Oct 21 2021 3 min read.
As of date India is. The Indian embassy on Wednesday informed that Oman has added Covaxin to the countrys approved list of Covid-19 vaccines which means Indians travelling to Oman will not be required to undergo quarantine upon arrival. As countries open their borders to travel tourism and education Bharat Biotechs Covaxin awaits WHOs nod on October 26.
The World Health Organizations WHO is yet to approve. The global health body has updated the decision date for Bharat Biotechs Covaxin as October 2021 on its latest EUL guidance document for Covid. In this regard the Strategic Advisory Group of Expert on Immunization SAGE will be meeting on October 5 to grant EUA to Covaxin.
Co-founder Suchitra Ella is hopeful of a positive answer. October 20 2021 1044 AM IST Updated Date. There is a procedure of submitting the documents for approval.
The Hyderabad-based company applied for WHO approval on 19 April by providing Expression of Interest EoI. Government ducks question on WHO Covaxin approval. The latest Status of COVID-19 vaccines within the WHO EULPQ evaluation process guidance document dated 29 September on the WHO website said that the decision date for Bharat Biotechs Covaxin is October 2021.
The WHO is currently reviewing the data submitted by the vaccine maker and the date for a decision on the jab is October 2021 according to the update available on the WHO website. The WHO said it began rolling data of the vaccine on 6 July. AFP Sajjad HUSSAIN.
WHO emergency approval which. So far the WHO has approved Covid vaccines developed by Pfizer-BioNTech US pharma majors Johnson Johnson Moderna Chinas Sinopharm and Oxford-AstraZeneca for emergency use. WHO to take final call on approval of Covaxin next week.
Aug 12 2021 Updated Aug 12 2021 953 AM IST. Covaxin is still to be authorised by Western authorities. The FDA rejected Bharat Biotechs proposal for emergency use approval of Covaxin the Covid-19 vaccine in the US because the company submitted partial trial data from March this year.
Decision on Covaxin Approval for Emergency Listing Due in October. Home News India WHO Reviewing Data on Bharat Biotechs Covaxin Decision on Approval in 24 Hours or So 4-MIN READ A health worker holds up Covaxin vials at a vaccination centre in Kolkata on June 22 2021. The WHO has approved COVID-19 vaccines by Pfizer-BioNTech AstraZeneca Johnson and Johnson Moderna and Sinopharm till date.
World Health Organisations WHO approval for the emergency use authorisation EUA to COVID-19 vaccine Covaxin developed by the. 15-year-old girl gang-raped for 9 months in Maharashtra 26 held. The WHO will take a call on granting Emergency Use Listing EUL status for Bharat Biotechs COVID-19 vaccine Covaxin next week the global health body said on Tuesday.
The Serum Institute-produced Covishield vaccine also received approval in January and has been used to inoculate the majority of vaccine recipients in India. So we would not like to comment on the date. Covax covaxin approval covaxin eul Covaxin who approval Covishield WHO Chief Published Date.
Covaxin the companys home-grown Covid-19 vaccine approved for emergency use in India is one of two shots driving the countrys massive vaccination programme launched on January 16. The Drugs Controller General of India DGCI had granted restricted emergency use approval for Covaxin in January. On June 4 Anvisa approved exceptional imports of Covaxin imposing conditions that restrict it mainly to healthy adults and limiting it to just 1 of the countrys population to manage the risks through control and supervision of side effects.
As of now the WHO has approved Covid vaccines by PfizerBioNTech Astrazeneca-SK BioSerum Institute of India AstraZeneca EU Janssen Moderna and Sinopharm for emergency use.

Fact Check Covaxin Has Not Been Approved For Children Above 12 Years Yet Fact Check News
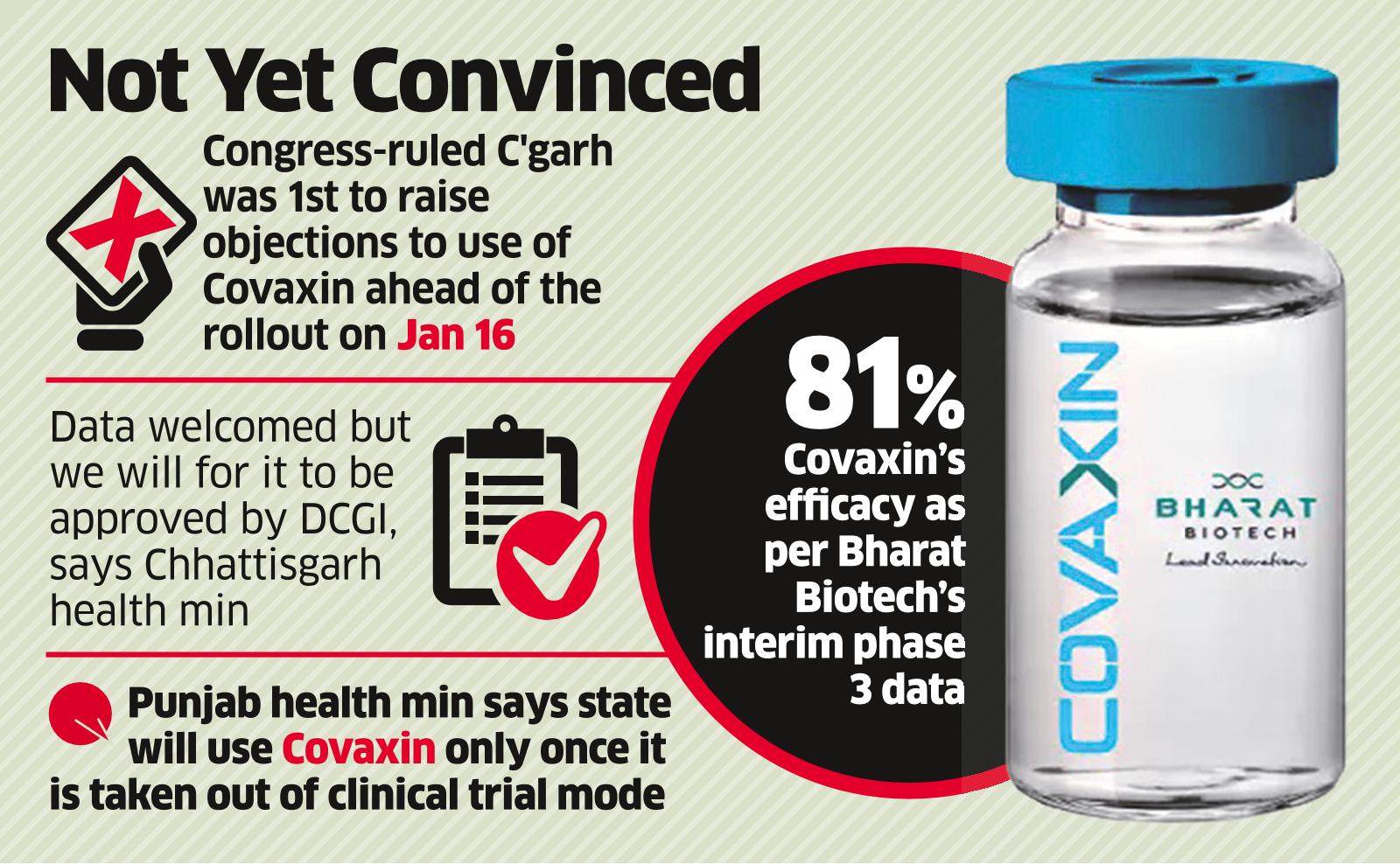
Covaxin Efficacy Data Has No Effect On Opposition States The Economic Times
Covaxin Vaccine India News Latest News Top Stories On Covaxin In India

Covaxin S Approval To Be Discussed On 5 Oct
Why Was Bharat Biotech S Covaxin Not Approved In Us Here S What We Know So Far Latest News India Hindustan Times

Who To Decide On Bharat Biotech S Emergency Approval For Covaxin In October
Coronavirus India Approves Covid 19 Vaccines Covishield And Covaxin For Emergency Use The Hindu

India Gets 2 Vaccines Against Coronavirus As Covaxin Covishield Get Final Nod By Dcgi

Covaxin May Soon Get Who Approval As Expert Group Scheduled To Meet Next Month

As Indians Who Took Covaxin Await Approval For Travel What S Causing Delay In Who Nod

When Can We Expect Who Approval For Covaxin

Covid 19 Covaxin Gets Shelf Life Extension Up To 12 Months The Financial Express
Covaxin May Get Who Approval By Month End Niti Aayog

Covaxin India S First Indigenous Covid 19 Vaccine Bharat Biotech

Covaxin Who Approval For Travel Status News Update More Updated November 2021 Wego Travel Blog
Delay In Covaxin S Approval Who Awaits Crucial Info

Covaxin For Children Making Trial Data Public Will Create Trust In Paediatric Use Vaccines The Financial Express

)



Post a Comment
Post a Comment