Covaxin Who Approval Status Today / Fact Check Covaxin Has Not Been Approved For Children Above 12 Years Yet Fact Check News
Covaxin gets WHO approval for Emergency Use Listing. Union Health Minister Mansukh Mandaviya on Tuesday said that approval for Hyderabad-based Bharat Biotechs COVID-19 vaccine Covaxin will be.
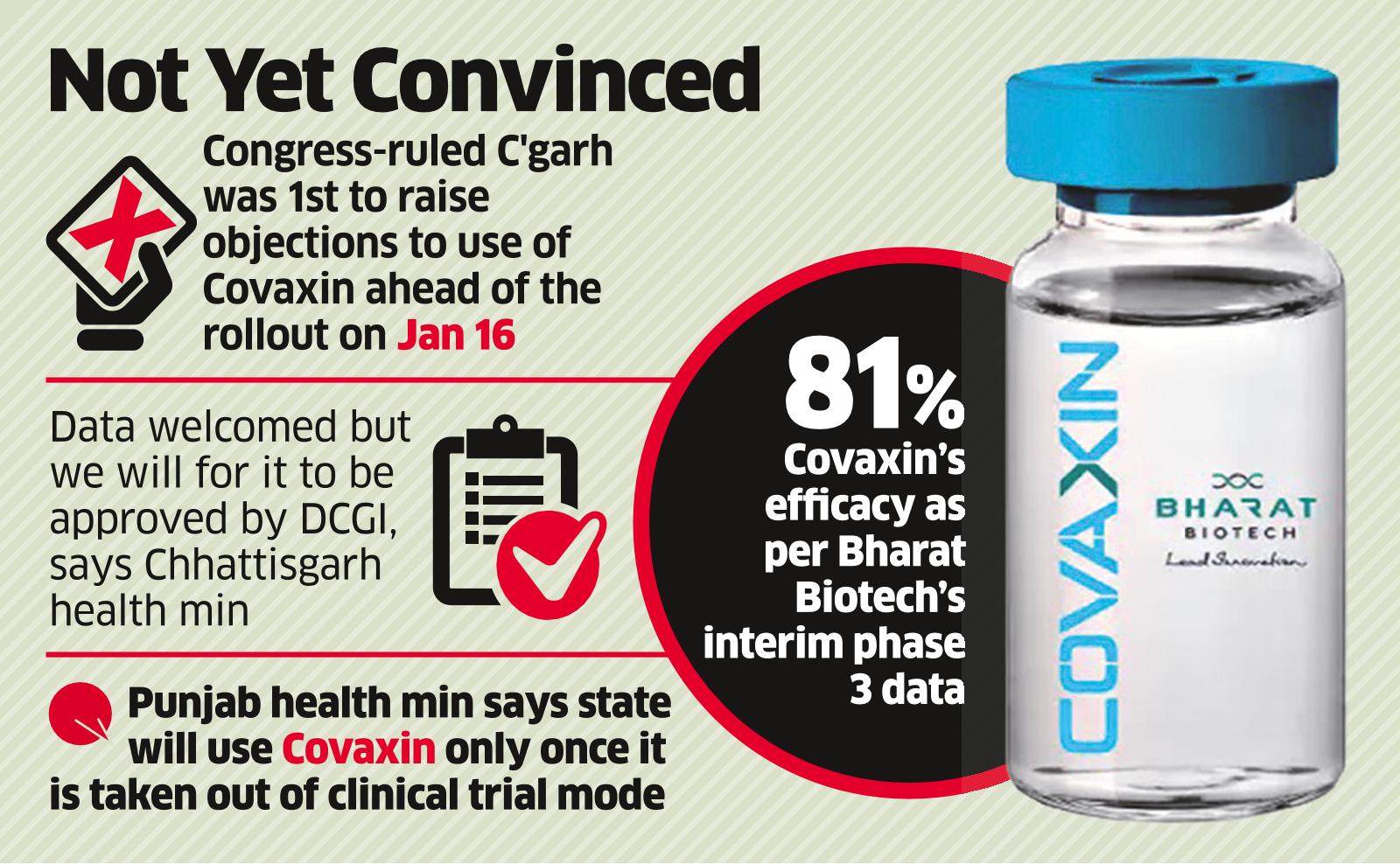
Covaxin Efficacy Data Has No Effect On Opposition States The Economic Times
Bharat Biotechs Covaxin is one of the six vaccines that have received emergency use authorisation from Indias drug regulator along with Covishield and Sputnik V.
Covaxin who approval status today. The Expression of Interest EOI for the emergency use approval of Covaxin was submitted by the company on April 19 2021 after which WHO demanded more data from the company. Bharat Biotechs Covaxin and AstraZeneca and Oxford Universitys Covishield are the two widely used vaccines in India. A decision on Covaxins inclusion in the Emergency Use List will be taken in 2-3 months time the Union government informed the Rajya Sabha on Tuesday.
Indias first indigenous COVID-19 vaccine Bharat Biotechs Covaxin was granted Emergency Use Listing EUL by the World Health Organization WHO on Wednesday. With this the WHO has approved six vaccines for the prevention of COVID-19. Indias indigenous Covid-19 vaccine Covaxin is in line to receive WHOs emergency use listing authorisation in October according to the world bodys document.
Bharat Biotech - the manufacturer of Covaxin - has been submitting data to WHO on a rolling basis and WHO experts have reviewed these data. The panel WHOs Technical Advisory Group for Emergency Use Listing TAG-EUL sought more clarification from the Covaxin. The Technical Advisory Group of the World Health Organisation WHO has recommended Emergency Use Listing status for Bharat Biotechs Covaxin news agency ANI reported on.
As part of the Phase IIIII trial the two-dose Covaxin was administered to 525 children 28 days apart. The WHO has given approval to Pfizer-BioNTech AstraZeneca-SK BioSerum Institute of India Johnson 7 Johnson Janssen. The foreign secretary further stressed that its a technical process and not an administrative or a political process.
Covaxin manufactured by Bharat Biotech India and BBIBP-CorV manufactured by Sinopharm China vaccines would be recognised for the purpose of establishing a travellers vaccination status. DCGI will now to take the final call. As of now the WHO has approved Covid vaccines by PfizerBioNTech Astrazeneca-SK BioSerum Institute of India AstraZeneca EU Janssen Moderna and Sinopharm for emergency use.
Foreign Secretary Harsh Vardhan Shringla has said that Bharat Biotechs Covaxin Indias first indigenous Covid-19 vaccine will be approved by the World Health Organisation WHO soon. Hyderabad-based pharmaceutical firm Bharat Biotech which is the company that developed Covaxin has been awaiting the EUL for the COVID-19 shot for months now. While some countries like Guyana Iran Mauritius Mexico Nepal Paraguay Philippines Zimbabwe Estonia which has approved the jab for its vaccine passport have already approved Covaxin with WHOs approval today many other countries will open their doors to citizens from India.
The Technical Advisory Group of the WHO recommended Emergency Use Listing status for Bharat Biotechs Covaxin. The nod could translate into the. Covaxin Bharat Biotechs COVID-19 vaccine has been granted Emergency Use Listing status by a World Health Organisation panel today.
As Bharat Biotech had submitted the data required for WHOs approval on July 9 2021 the process is. The World Health Organization WHO today granted the emergency use listing EUL status to Covaxin which has been developed by Bharat Biotech in India. Read more about Bharat Biotechs Covaxin gets WHO approval for Emergency Use Listing on Business Standard.
Bharat Biotech had received the drug regulators nod to conduct the childrens trial in May this year. The WHO has so far approved Covid-19 vaccines of Pfizer-BioNTech AstraZeneca-SK Bio Serum Institute of India Johnson Johnson-Janssen Moderna and Sinopharm for. The World Health Organization today approved Indias indigenous Covid-19 vaccine Covaxin for emergency use listing EUL status.
This recognition is for travellers aged 12 and over who have been vaccinated with Covaxin and those 18 to 60 who have been vaccinated with BBIBP-CorV a media release from the Australian. WHO panel likely to discuss emergency use of Covaxin today. Covaxin was reviewed by WHOs Strategic Advisory Group of Experts on Immunization and recommended use of this vaccine in two doses with a dose interval of four weeks in all age groups 18 and above.
Earlier today Bharat Biotech said the Central Drugs Standard Control Organisation CDSCO has approved the extension of shelf life of its COVID-19 vaccine Covaxin. In addition it is also likely to ease travel for those Indians who wish to travel due to education work or. New Delhi October 3.
The World Health Organisations WHO approval for the emergency use authorisation EUA to COVID-19 vaccine Covaxin developed by the Hyderabad-based Bharat Biotech is. Earlier the company had earlier indicated that if all goes well the vaccine for kids would be rolled out in October itselfCovaxin. Covaxin developed by Bharat Biotech was granted the.
Subject Expert Committee has approved Bharat Biotechs covid vaccine COVAXIN for children in the 2-18 age group.

Covid 19 Vaccine Covaxin Approval Who Seeks More Data From Bharat Biotech For Emergency Use Licensing The Financial Express

Fact Check Covaxin Has Not Been Approved For Children Above 12 Years Yet Fact Check News

Covaxin Approval Soon Who Chief Scientist Says Phase 3 Trial Data Looks Good Breaking News Youtube

Covaxin Bharat Biotech S India Made Covid Vaccine Cleared By Who Sources

Covaxin Added To Approved List Of Covid Vaccines For Travel To Oman Without Quarantine India News India Tv

Covaxin Who Approval For Travel Status News Update More Updated November 2021 Wego Travel Blog

Who Grants Approval To Covaxin For Emergency Use Listing

Covaxin Approval Today Crucial W H O Meet Today To Decide On Nod For Bharat Biotech S Covaxin Youtube

Who Seeks Additional Clarifications For Covaxin Approval Final Assessment Of Bharat Biotech S Covid Vaccine On November 3 India News

After Who Approval Us To Allow Travellers Vaccinated With Covaxin To Enter Country From Nov 8 Coronavirus Outbreak News

Bharat Biotech S Covaxin Likely To Get Who S Emergency Use Approval Today

Who Meet On Covaxin Emergency Use Approval Today Decision Likely Within 24 Hours India News Zee News
Coronavirus India Approves Covid 19 Vaccines Covishield And Covaxin For Emergency Use The Hindu





Post a Comment
Post a Comment